Cell and gene therapies: Pharma's next big wave
Leveraging the untapped potential of cell and gene therapies
"Cell and gene therapies not only have huge potential to save lives, but also significant economic potential."
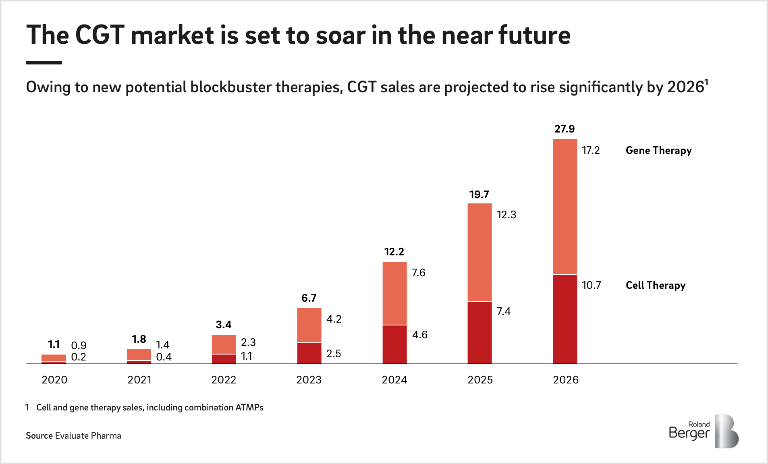
With the potential to cure serious diseases like cancer and persistent virus infections, Cell and Gene Therapies (CGT) are poised to transform the pharmaceutical and biotech industry soon. The market is projected to grow rapidly, reaching sales forecasts of EUR 27.9 billion by 2026. There is little doubt that cell and gene therapies are a promising new segment, but market entry is not straightforward, and established pharma & biotech companies must develop new strategies to enter the market and become respected players. This is the finding of our latest study, ‘Pharma’s big opportunity to ride the next wave’, which explores the potential of these new therapies and the major considerations for CGT entrants.
"Cell and gene therapies have come a long way – they are no longer science fiction as the underlying technology platforms are tried and tested by now."
Gaining access to the booming CGT market
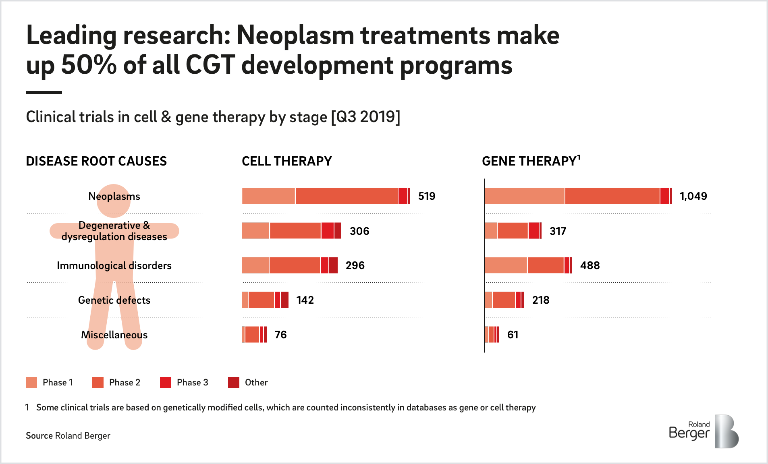
CGT is an exciting, innovative field that has the potential to permanently change the course of debilitating human diseases, such as cancer or genetic disorders. Within this sector, two distinct areas of focus have emerged. Gene therapy (gene editing and augmentation) replaces or repairs DNA to help the body regain functions or prevent dysfunctions caused by diseases, genetic disorders or viruses. One form of gene therapy includes a CRISPR-based treatment, that precisely cuts DNA with "gene scissors" to correct a genetic defect. Cell therapy, on the other hand, uses specially grown or adapted human cells (from the patient or a donor) to replace depleted tissue or perform therapeutic functions. Common types of cell therapy treatments include stem cell transplant, which can create bone marrow, and blood transfusion.
Acquiring stakes in both clinical areas of therapy is intense, competitive and involves huge sums, with deals regularly topping the billion-dollar mark. For example, Gilead bought Kite Pharma for USD 12 billion in 2017. Nonetheless, companies that jump onto the CGT bandwagon now may reap great rewards later. While there are only few products on the market so far, the US Food and Drug Administration (FDA) expects to license at least 10 cell therapy and gene therapy products per year starting in 2025.
"The right to win in CGT comes with proficiency in the underlying platforms from science to patient."
Since cell and gene therapy is a completely new field of technology, pre-existing knowledge on the subject is limited, and the chance of discovering something monumental is real. Companies currently involved in the science, technology and clinical trials behind these therapeutics have already achieved valuations of several billion dollars before generating their first revenues. Sales are expected to keep climbing, with gene therapy predicted to bring in EUR 17.2 billion by 2026, and cell therapy EUR 10.7 billion.
Notwithstanding the above, successfully entering this novel field of cells and genes requires more than a willingness to take risk. Pharma players need to soundly prepare both from the science and technology end as well as from the organizational and cultural end .
Five key priority areas
Roland Berger developed the following five key considerations and recommendations to help pharma & biotech companies wishing to get involved:
- Research and development: Full control and continuous development of the underlying technology platform is of utmost strategic importance. Following this path, companies can stay ahead of their competition when it comes to any advancements in the study of cells, genes and genetics.
- Business development and licensing: Rapid evaluation of acquisitions targets in this market – in weeks, not months – is a basic requirement. The emphasis should be on science without too many checkpoints and stage gates to cross.
- Manufacturing: Special attention to the production process is crucial for new players entering the field. Owning the entire end-to-end manufacturing and supply chain process should be the goal.
- Market access, marketing and sales: Early interaction with regulators and payors will enable the fast roll out of new therapies and clinical trials. Being first-to-market in rare diseases is important, as is the patient benefit.
- Organization, governance and culture: Embracing novel platform technologies within CGT next to existing platforms of small and large molecules poses challenges to established ways of working. Strong commitment and a play-to-win attitude are required for success.
Register now to download the full study, including all insights into the cell and gene therapy market and what recommendations we can give you when entering this potential market.